2x1 Double-Clad Fiber Couplers
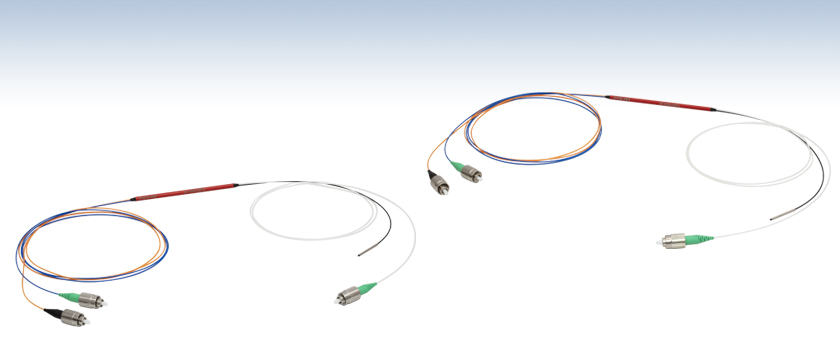
- Small Cladding, Bidirectional, and High Extraction 2x1 DCF Couplers
- 1260 - 1600 nm Wavelength Range
- Single Mode Core Insertion Loss of ≤0.5 dB
- At Least 70% Multimode Inner Cladding Transfer
DC1300SEFA
Small Cladding Double-Clad Fiber
DC1300LE2FA
High Extraction Double-Clad Fiber

Please Wait
Click to Enlarge
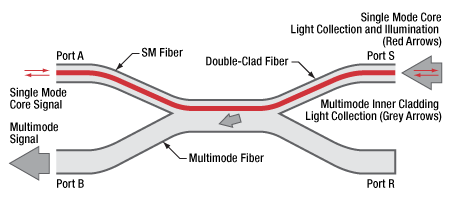
The schematic above shows the internal structure of our high-extraction double-clad fiber coupler. Single mode light input at Port A is used to illuminate a sample at Port S. Single mode and multimode light from the sample enters the single mode core (shown in red) and multimode inner cladding (shown in grey) of the DCF at Port S. The single mode signal travels through the core of the DCF and is output at port A; the multimode signal is transferred from the DCF to the multimode fiber and is output at Port B.
Applications
- Optical Coherence Tomography (OCT)
- Fluorescence Imaging
- Confocal Microscopy
- Spectroscopy
- Surface Plasmon Resonance (SPR) Sensing
- Speckle-Free Single-Fiber Endoscopy
- LIDAR

Fiber leads containing SM fiber are color-coded blue (Port A), orange for multimode fiber (Port B), white for double-clad fiber (Port S), and black for the terminated port (Port S).
Features
- 1260 - 1600 nm Wavelength Range
- Small Cladding Coupler Allows Quasi-Confocal Signal Collection
- Bidirectional Coupler Allows Port B to be Used as Both an Input and Output
- High Extraction Coupler has an Extraction Ratio of up to 85 - 90%
- ≤0.5 dB Single Mode Core Insertion Loss
- At Least 70% Multimode Inner Cladding Transfer
- Individualized Data Sheet Included with Each Coupler (See the Verification Tab; Sample Data Sheet Available for Small Cladding, Bidirectional, and High Extraction Couplers)
Thorlabs is collaborating with strategic partner Castor Optics to design and manufacture a family of Double-Clad Fiber Couplers. These 2x1 Double-Clad Fiber Couplers combine a double-clad fiber (single mode core surrounded by a multimode inner cladding) with a standard single mode or step-index multimode fiber, as shown in the illustration to the right for the DC1300LE2FA high extraction coupler. The DC1300SEFA coupler allows a quasi-confocal signal collection provided by its small inner cladding. The DC1300LQ2FA coupler is bidirectional, allowing port B to be used as an input or an output. The DC1300LE2FA coupler has a high extraction ratio of up to 85 - 90%. Please see the Applications tab for more information.
Operation
Light in the single mode core transmits with ≤0.5 dB core insertion loss over the 1260 - 1600 nm wavelength range. The multimode transfer, defined as the ratio of the output signal at Port B to the input signal at Port S, is ≥70% over the bandwidth for the DC1300SEFA coupler, and ≥85% for the DC1300LE2FA coupler, in both cases excluding the water absorption region around 1383 nm. The DC1300LQ2A coupler is bidirectional, and has a multimode transfer of ≥70% from port S to port B, but a transfer of ≥55% from port B to port S. The WMC2L1F wideband multimode circulator can be used at Port B of the bidirectional coupler to use that port both as input and output, enabling concurrent multimode illumination and collection. Using the two together allows for the combination of OCT and hyperspectral imaging.
In order to minimize the single mode (SM) connection losses between an SM system and the double-clad fiber, SMF-28 fiber is spliced into each coupler at port A. The diagram to the top right depicts both single mode and multimode signals through a high-extraction DCF coupler. For additional schematics, please refer to the info icons
(![]() ) below.
) below.
Applications
The double-clad fiber couplers function as an alternative to free-space assemblies in many applications, including imaging and sensing. The video below to the right compares images obtained from the single mode and multimode outputs of a 1300 nm double-clad fiber coupler.
These couplers with a Ø105 µm inner cladding have been used to combine OCT imaging with fluorescence detection, as well as in speckle-free single fiber endoscopy applications, and are appropriate for LIDAR and free-space communication applications due to their wavelength range extending to 1550 nm.
Construction
The fiber in each leg is jacketed in a Ø900 µm Hytrel®* tube, color-coded blue for the SMF-28 leg (Port A), white for the DCF leg (Port S) and orange for the MM fiber leg (Port B), as shown in the schematic to the right. Port R, color-coded black, is terminated based on a proprietary process optimizing the return loss performance for high-sensitivity applications such as long-range telemetry.
These couplers are housed inside of a protective tube and are available with two 2.0 mm narrow key FC/PC and one 2.0 mm narrow key FC/APC connectors. In general, FC/APC connectors reduce back reflections from the single mode core of the double-clad fiber leg of the coupler; reflection is not an issue for the multimode output port, so an FC/PC connector is used for easier integration with other fiber components.
Custom connector configurations and different performance characteristics may be available. Additionally, the double-clad fiber used in the 1300 nm coupler is available from stock (Item # DCF13) and in a patch cable upon request; please contact info@castoroptics.com with all custom inquiries. 2x2 versions of the double-clad fiber couplers are also available here.
*Hytrel® is a registered trademark of DuPont Polymers, Inc.
| Webpage Features | |
|---|---|
| |
Clicking this icon below will open a window that contains specifications and drawings. |
Double-Clad Fiber Coupler (DCFC) Applications
Double-Clad Fiber Couplers can be implemented in many imaging, sensing, communication, and LIDAR applications. The examples below specifically illustrate how DCFCs can be used in confocal microscopy, OCT, and endoscopy applications. For more implementations and practical considerations, please refer to the following article.
Click to Enlarge
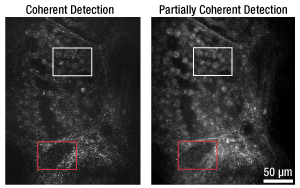
Figure 1. Comparison of confocal images taken using a single mode fiber coupler (left) and DC780SEFA DCFC (right).
Confocal Microscopy and Partially Coherent Detectiona
In a free-space confocal microscope, pinhole apertures only allow coherent light at the focal plane to reach the detector, which enables optical sectioning and high-resolution images over a narrow focal plane. Widening the detection aperture allows a small amount of partially coherent light to reach the detector, creating images with reduced speckle noise and increased contrast, but slightly reduced resolution.
This same effect can be achieved using a small-diameter DCFC, such as the DC780SEFA or DC1300SEFA. In this scenario, the single mode core acts as an illumination pinhole while the small inner cladding serves the purpose of the detection pinhole. Together, the core and inner cladding enable optical sectioning of the image just as in free space confocal microscopy. Using a DCFC in this manner ensures that the pinholes are always conjugate, because the detection pinhole surrounds the illumination pinhole. Finally, because the inner cladding diameter is just slightly larger than the core diameter, a small amount of partially coherent light is accepted, which reduces speckle noise and increases contrast.
Figure 1 compares images taken of a swine thyroid tissue section using a 50:50 single mode fiber coupler and a DC780SEFA DCFC, respectively. As seen in comparing the white and red boxed sections in the left and right images, using a DCFC reduces speckle artifacts in the image while increasing contrast of cellular features within the tissue sample. Figure 2 shows a series of confocal images taken of swine muscle tissue at depths from 15 µm to 105 µm using a single mode fiber coupler and a DC780SEB double-clad fiber coupler.
Optical Coherence Tomography and Fluorescence Imagingb

Click to Enlarge
Figure 5. Combined OCT and Fluorescence Image
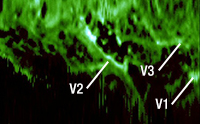
Click to Enlarge
Figure 4. Fluorescence Image
Click to Enlarge
Figure 3. OCT Image
DCFCs can also be used instead of traditional dichroic beamsplitters to allow both OCT and fluorescence imaging of a sample using the same objective and scanning optics. The OCT signal (Figure 3) is collected in the single mode core of the double-clad fiber (DCF), while the fluorescence signal (Figure 4) is collected in the cladding. The core and cladding signals can be combined to produce a detailed image that distinguishes between bronchioles and blood vessels, as shown in Figure 5.

Click to Enlarge
Figure 6. Courtesy of Xavier Attendu
Optical Coherence Tomography and Multispectral Imaging
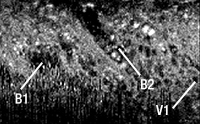
Bidirectional DCFCs enable the combination of coherent imaging, such as OCT, with a modality requiring diffuse illumination. An exemplary application is the combination of OCT with multispectral imaging. This combination allows clinicians to access in-depth structural information provided by OCT images, overlaid with true-color data extracted from multispectral acquisition. For example, combining a bidirectional DCFC, such as the DC1300LQ2FA, with the WMC2L1F multimode circulator provides a highly flexible illumination system: light can be either injected in the SM core for high resolution illumination or in the DCF’s inner cladding for significant signal increase. Figure 6 presents multispectral images of printed logos (true image in A) when illuminated with the SM core (B) versus the DCF’s inner cladding (C). In both cases, the collection is performed with the large inner cladding to ensure optimal signal-to-noise ratio.
References
Double-Clad Fiber Coupler Verification
Our double-clad fiber couplers undergo stringent verification testing during production. The setups shown below are used to obtain a single mode transmission spectrum, insertion loss, and the multimode inner cladding transfer specification. Each coupler is shipped with an individualized data sheet providing a summary of the results of these tests. Click for a sample data sheet for Small Cladding, Bi-Directional, and High Extraction Couplers.
Click to Enlarge
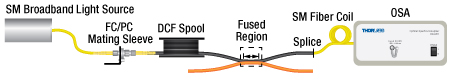
Single Mode Insertion Loss and Transmission Measurement
Step 1: Single Mode Insertion Loss/Transmission Measurement
The single mode input of the coupler is connected to a Broadband Light Source (BBS) through a single mode fiber and a spool of double-clad fiber (DCF). The SM coupler output is spliced to a coiled SM patch cable (to ensure cladding modes are stripped) that is connected to an Optical Spectrum Analyzer (OSA). A spectrum is recorded before and after the fibers are fused to create the coupler. The difference between the two spectra can be defined as either Insertion Loss (dB) or Transmission (%).
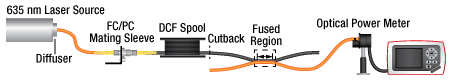
Step 2: Multimode Transfer Measurement
Click to Enlarge
Multimode Transfer Measurement
The multimode input of the coupler is connected to a diffused 635 nm laser source through a Ø105 µm core / Ø125 μm cladding multimode fiber and a spool of DCF. Doing so ensures that the inner cladding modes are filled. The Ø200 µm core / Ø220 μm cladding multimode fiber output of the coupler is connected to a silicon photodiode optical power meter. A first optical power is recorded. The coupler is then removed from the measurement setup and the DCF spool is connected directly to the same power meter. A second optical power is recorded. The Multimode Inner Cladding Transfer is defined as the ratio of the first to second power measurements (%).

Castor Optics, a Montreal-based leading manufacturer of specialized fiber optics, has been a key strategic partner of Thorlabs since 2013. Castor was founded by Caroline Boudoux, Nicolas Godbout, Normand Brais, and Alex Cable to commercialize the innovative fiber coupler technology developed in the laboratory by Caroline Boudoux and Nicolas Godbout (Polytechnique Montreal). The team at Castor works closely with Thorlabs' Montreal office to develop exclusive and innovative fiber optics products and processes.
In addition to their co-presidents, Professors Boudoux and Godbout, Castor is driven by a team of fiber optics experts, engineers, and scientists. Leveraging its world-class fiber optics components development expertise, Castor pursues its mission to bring innovative solutions to the market to cater to industries requiring high-sensitivity performances, such as biomedical sensing, free-space communications, industrial sensing, and defense and security. They provide continuing support through their customers’ journey from prototyping to integration and manufacturing.

Click to Enlarge
The Castor Team in their Montreal Facility
Review Article
K. Beaudette, J. Li, J. Lamarre, L. Majeau, and C. Boudoux. “Double-Clad Fiber-Based Multifunctional Biosensors and Multimodal Bioimaging Systems: Technology and Applications,” Biosensors, 12(2):90, (2022).
K. Beaudette, N. Godbout, and C. Boudoux. "Advances in Multimodal Imaging Using Double-Clad Fiber Couplers," J. Lightwave Technol. 37, 5674-5685 (2019).
Combined OCT and Fluorescence Imaging
D. Lorenser, B. C. Quirk, M. Auger,W.-J. Madore, R.W. Kirk, N. Godbout, D. D. Sampson, C. Boudoux, and R. A. McLaughlin. "Dual-modality needle probe for combined fluorescence imaging and three-dimensional
optical coherence tomography," Optics Letters, 38, 266 - 268 (2013).
L. Scolaro, D. Lorenser, W. J. Madore, R. W. Kirk, A. S. Kramer, G. C. Yeoh, N. Godbout, D. D. Sampson, C. Boudoux, and R. A. McLaughlin, "Molecular imaging needles: dual-modality optical coherence tomography and fluorescence imaging of labeled antibodies deep in tissue," Biomed. Opt. Express, 6, 1767-1781 (2015).
H. Pahlevaninezhad, A. M. D. Lee, G. Hohert, T. Shaipanich, E. L. Beaudoin, C. MacAulay, C. Boudoux, and P. Lane, "Endoscopic high-resolution autofluorescence imaging and OCT of pulmonary vascular networks," Optice Letters, 41, 3209-3212 (2016).
OCT and Laser Therapy
R. Maltais-Tariant, C. Boudoux, and N. Uribe-Patarroyo, "Real-time co-localized OCT surveillance of laser therapy using motion corrected speckle decorrelation," Biomed. Opt. Express, 11, 2925-2950 (2020).
K. Beaudette, H. Won Baac, W. J. Madore, M. Villiger, N. Godbout, B. E. Bouma, and C. Boudoux, "Laser tissue coagulation and concurrent optical coherence tomography through a double-clad fiber coupler," Biomed. Opt. Express, 6, 1293-1303 (2015).
OCT and Multispectral Imaging
X. Attendu, M. H. Bourget, M. Poinsinet de Sivry-Houle, C. Boudoux, "Coregistered optical coherence tomography and frequency-encoded multispectral imaging for spectrally sparse color imaging," J. Biomed. Opt. 25(3) 032008 (2019).
R. Guay-Lord, X. Attendu, K. L. Lurie, L. Majeau, N. Godbout, A. K. Bowden, M. Strupler, C. Boudoux, "Combined optical coherence tomography and hyperspectral imaging using a double-clad fiber coupler," J. Biomed. Opt. 21(11) 116008 (2016).
OCT and SLO
J. D. Malone, M. T. El-Haddad, I. Bozic, L. A. Tye, L. Majeau, N. Godbout, A. M. Rollins, C. Boudoux, K. M. Joos, S. N. Patel, and Y. K. Tao, "Simultaneous multimodal ophthalmic imaging using swept-source spectrally encoded scanning laser ophthalmoscopy and optical coherence tomography," Biomed. Opt. Express, 8, 193-206 (2017).
| Posted Comments: | |
| No Comments Posted |

| Item # | Info | SM Wavelength Range |
Core Insertion Lossa,b (Click for Plot) |
MM Wavelength Range |
MM Inner Cladding Transfera,c |
Port A Fiber | Port B Fiber | Port S Fiber | Terminationd (Click for Diagram) |
|---|---|---|---|---|---|---|---|---|---|
| DC1300SEFA | 1260 - 1600 nm | ≤0.5 dB | 400 - 2200 nm | ≥70% (Port S to B) | SMF-28 | FG200LEA | DCF1300S-41 | Ports A and S: FC/APC Port B: FC/PC Port R: Terminated |

| Item # | Info | SM Wavelength Range |
Core Insertion Lossa,b (Click for Plot) |
MM Wavelength Range |
MM Inner Cladding Transfera,c |
Port A FIber | Port B Fiber | Port S Fiber | Terminationc (Click for Diagram) |
|---|---|---|---|---|---|---|---|---|---|
| DC1300LQ2FA | 1260 - 1600 nm | ≤0.5 dB | 400 - 2200 nm | ≥75% (Port S to B) ≥55% (Port B to S) |
SMF-28 | FG105LCA | DCF13 | Ports A and S: FC/APC Port B: FC/PC Port R: Terminated |

| Item # | Info | SM Wavelength Range |
Core Insertion Lossa,b (Click for Plot) |
MM Wavelength Range |
MM Inner Cladding Transfera,c |
Port A Fiber | Port B Fiber | Port S Fiber | Terminationc (Click for Diagram) |
|---|---|---|---|---|---|---|---|---|---|
| DC1300LE2FA | 1260 - 1600 nm | ≤0.5 dB | 400 - 2200 nm | ≥85% (Port S to B) | SMF-28 | FG200LEA | DCF13 | Ports A and S: FC/APC Port B: FC/PC Port R: Terminated |
 Products Home
Products Home









 Double-Clad 2x1 Fiber Couplers
Double-Clad 2x1 Fiber Couplers