Transmitted Light Illumination for DIY Cerna® Systems
- Illuminate Thin Specimens Through a Condenser
- Module Conditions Sample Illumination
- Compatible with Collimated Light Sources in 30 mm
Cage Systems
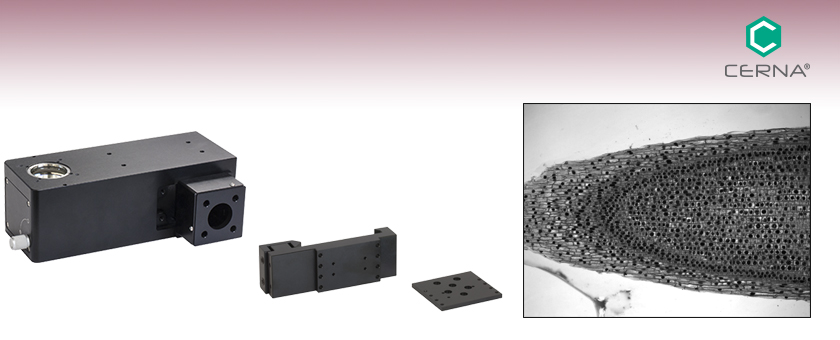
WFA1000
Illumination Module
WFA0150
Dovetail Clamp
Brightfield Image of Onion Mitosis

Please Wait

Click to Enlarge
The WFA1000 Illumination Module is positioned in the optical path by the WFA0150 Dovetail Clamp.

Click to Enlarge
Our trans-illumination modules accept collimated illumination sources that have been mounted in a 30 mm cage system. The optical output port is also 30 mm cage compatible; here, it is shown holding our CRM1T Rotation Mount and a polarizer.
Click for Details
Drawing of Trans-Illumination Module
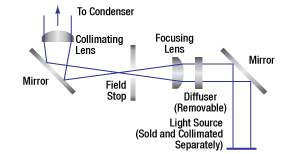
Click to Enlarge
Trans-Illumination Module Schematic
Features
- Module Accepts Collimated Illumination Through Ø1" Optical Input Port
- Optical Input and Output Ports are 30 mm Cage Compatible
- 95 mm Dovetail Clamp Attaches Module to Microscope Body
- Compatible with Brightfield, Oblique, and Darkfield Illumination, as well as Other Modalities
- Designed for 7.74" Throat Depth of DIY Cerna® Systems
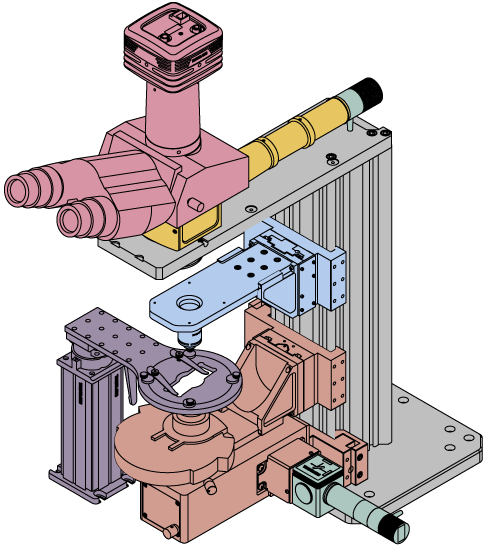
Thorlabs' WFA1000 Trans-Illumination Module steers the light from a user-provided illumination source into the transmitted light optical path of a DIY Cerna system. Designed for use in the 400 - 1000 nm wavelength range, it is suitable for several imaging modalities that transmit light through thin samples, such as brightfield, oblique, and darkfield illumination. It can also be adapted for differential interference contrast (DIC) imaging using our DIC accessories.
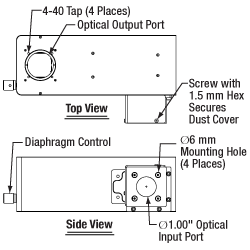
As shown in the drawings to the right, the WFA1000 module accepts collimated illumination through a Ø1" input port on the side of the module. To mount an illumination source to this port, the module accepts cage rods via four Ø6 mm bores spaced for our 30 mm cage system. Locking setscrews for these cage rods can be accessed by removing a dust cover that is held in place with a 1.5 mm hex button head screw, as shown in the drawing to the right. Our illumination kits are specifically designed for use with this input port, and four ER025 cage rods for these kits are included with the module. If using another light source, note that for best performance, the input port should be filled.
The rotating knob on the front controls an integrated field stop diaphragm, which can be used to adjust the illumination intensity and match the back aperture of a condenser. A diffuser comes pre-installed inside the module in order to homogenize the output light. If desired, the user can access and easily remove this diffuser by unscrewing four #1 Phillips-head screws on the side of the module. 30 mm cage compatibility for the output port is provided by four 4-40 taps.
The WFA0150 Dovetail Clamp, sold separately, is used to connect the trans-illumination module to the microscope body. This 95 mm dovetail clamp is attached to the trans-illumination module by the included adapter plate, as shown in the image to the right. The clamp and adapter plate together position the optical output port at the 7.74" throat depth used in DIY Cerna systems.
To complement our trans-illumination modules, Thorlabs offers several condensers that collect the output light to illuminate a specimen. All of these condensers enable brightfield illumination, and in addition, the CSC1001 Condenser ships with a mask for oblique illumination.
Other Transmitted Light Imaging Modalities
We also support Dodt contrast and DIC for DIY Cerna systems. Compared to brightfield illumination, these imaging modalities require more alignment and optical elements, but are better at obtaining contrast in thin samples. For more details, see the Imaging Modalities tab above.
Cerna® microscopes support several imaging modalities, including epi-fluorescence, brightfield illumination, differential interference contrast (DIC) imaging, and Dodt gradient contrast imaging. Each of these methods requires different accessories and confers different advantages to the microscopist, as described below.
Click to Enlarge
Epi-Fluorescence Image of Mouse Kidney with Multiple Labels
Epi-Fluorescence
Epi-fluorescence makes use of fluorescent labels and intrinsic fluorescence in a specimen to identify sample features. To create an epi-fluorescence image, light that has been passed through an excitation filter is directed through an objective and absorbed by a sample. This excitation causes fluorophores within the sample to emit light of a longer wavelength (i.e., lower energy) than the excitation light. Some of this emitted light is collected by the objective, which helps direct the emission onto a camera for observation. Additional details on this imaging modality are available here.
For performing epi-fluorescence measurements in DIY Cerna systems, we offer a range of widefield viewing and epi-illumination accessories, as well as fluorescence filter sets targeted at common fluorophores.
Click for Details
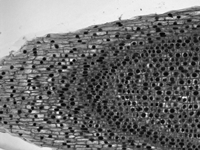
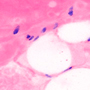
Brightfield Image of Onion Mitosis
Brightfield Illumination
Brightfield illumination is the simplest method of trans-illumination. In this modality, light from an illumination source is collected by a condenser and passed through a sample, which is observed by its effect on the intensity of the transmitted light. Brightfield illumination only requires an illumination source (i.e., an illumination kit) and a condenser to be attached to a DIY Cerna system.

Click to Enlarge
DIC Image of a Buttercup Root
DIC Imaging
In differential interference contrast (DIC) imaging, light transmitted through the sample is manipulated by a number of polarization optics. Light from the illumination source is polarized and then split into two orthogonally polarized beams before it reaches the sample. Small differences in the optical path length experienced by the two beams cause interference when the beams are recombined, providing enhanced contrast for sample features that would be transparent under brightfield illumination. In addition to an illumination source and a condenser, DIC imaging requires several additional optical elements: a DIC polarizer, a condenser prism, an objective prism, and an analyzer.

Click to Enlarge
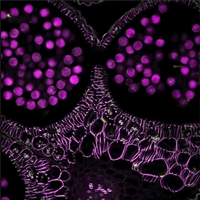
Dodt Contrast Image of a Mouse Retina
Dodt Contrast
Dodt gradient contrast, also known more simply as Dodt contrast, can be understood as an improvement upon oblique illumination. Both methods use a mask to generate an illumination gradient, but in Dodt contrast, the mask occurs much earlier in the optical path. This configuration improves the image contrast to a point where it is comparable to that obtained using DIC.
The Dodt illumination gradient is generated using a specially shaped quarter annulus and diffusers, and reveals thickness changes in a sample over the field of view. Compared to brightfield illumination, Dodt contrast offers improved resolution of sample features, and compared to DIC, it allows thicker samples to be studied. Thorlabs manufactures a pre-configured, pre-aligned illumination module for Dodt contrast that generates the desired gradient; it requires an illumination source and a condenser for operation.
Click to Enlarge
Laser Scanned Image of a Flower Bud
Laser Scanning
Like epi-fluorescence, laser scanning makes use of fluorescent labels and intrinsic fluorescence in a specimen to identify sample features. Unlike epi-fluorescence, laser scanning is able to resolve thin individual planes relatively deep into a thick sample, enabling 3D volumetric images and opening the door to in vivo studies.
Laser scanning techniques (e.g., multiphoton and confocal microscopy) rely upon the coherence of laser beams to provide significantly improved axial resolution. In confocal microscopy, a pinhole eliminates the out-of-focus light that would reduce the axial resolution (as it does in epi-fluorescence), while in multiphoton microscopy, the necessity of two- or three-photon absorption by the fluorophore, a low-probability event, effectively creates optical sections.
Additional details are available at our laser scanning microscopy tutorial.
Building a Cerna® Microscope
The Cerna microscopy platform's large working volume and system of dovetails make it straightforward to connect and position the components of the microscope. This flexibility enables simple and stable set up of a preconfigured microscope, and provides easy paths for later upgrades and modification. See below for a couple examples of the assembly of some DIY Cerna microscopes.
DIY Cerna Design and Assembly
Walkthrough of a DIY Microscope Configuration
This DIY microscope uses a CSA3000(/M) Breadboard Top, a CSA2001 Dovetail Adapter, our CSA1001 and CSA1002 Fixed Arms, and other body attachments and extensions. These components provide interfaces to our lens tube and cage construction systems, allowing the rig to incorporate two independent trans-illumination modules, a home-built epi-illumination path, and a custom sample viewing optical path.
The simplicity of Thorlabs optomechanical interfaces allows a custom DIY microscope to be quickly assembled and reconfigured for custom imaging applications.
| Posted Comments: | |
user
(posted 2024-05-03 15:00:10.527) Is it possible to adjust or recenter the outgoing light from the top of the illumination module? cdolbashian
(posted 2024-05-10 05:06:39.0) Thank you for reaching out to us with this inquiry. The only degrees of freedom which this component has is the ability to rotate the whole assembly about the optical path, and the ability to change the fine focal position of the image plane. I have contacted you directly to discover the exact issue you are having. Perhaps it can be solved elsewhere in your optical path. |
Click on the different parts of the microscope to explore their functions.
Elements of a Microscope
This overview was developed to provide a general understanding of a Cerna® microscope. Click on the different portions of the microscope graphic to the right or use the links below to learn how a Cerna microscope visualizes a sample.
Terminology
Arm: Holds components in the optical path of the microscope.
Bayonet Mount: A form of mechanical attachment with tabs on the male end that fit into L-shaped slots on the female end.
Bellows: A tube with accordion-shaped rubber sides for a flexible, light-tight extension between the microscope body and the objective.
Breadboard: A flat structure with regularly spaced tapped holes for DIY construction.
Dovetail: A form of mechanical attachment for many microscopy components. A linear dovetail allows flexible positioning along one dimension before being locked down, while a circular dovetail secures the component in one position. See the Microscope Dovetails tab or here for details.
Epi-Illumination: Illumination on the same side of the sample as the viewing apparatus. Epi-fluorescence, reflected light, and confocal microscopy are some examples of imaging modalities that utilize epi-illumination.
Filter Cube: A cube that holds filters and other optical elements at the correct orientations for microscopy. For example, filter cubes are essential for fluorescence microscopy and reflected light microscopy.
Köhler Illumination: A method of illumination that utilizes various optical elements to defocus and flatten the intensity of light across the field of view in the sample plane. A condenser and light collimator are necessary for this technique.
Nosepiece: A type of arm used to hold the microscope objective in the optical path of the microscope.
Optical Path: The path light follows through the microscope.
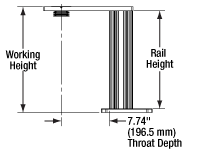
Rail Height: The height of the support rail of the microscope body.
Throat Depth: The distance from the vertical portion of the optical path to the edge of the support rail of the microscope body. The size of the throat depth, along with the working height, determine the working space available for microscopy.
Trans-Illumination: Illumination on the opposite side of the sample as the viewing apparatus. Brightfield, differential interference contrast (DIC), Dodt gradient contrast, and darkfield microscopy are some examples of imaging modalities that utilize trans-illumination.
Working Height: The height of the support rail of the microscope body plus the height of the base. The size of the working height, along with the throat depth, determine the working space available for microscopy.
 Click to Enlarge
Click to EnlargeCerna Microscope Body
Click to Enlarge
Body Details
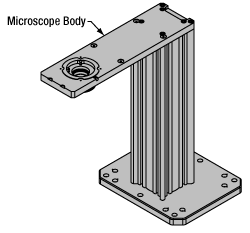
Microscope Body
The microscope body provides the foundation of any Cerna microscope. The support rail utilizes 95 mm rails machined to a high angular tolerance to ensure an aligned optical path and perpendicularity with the optical table. The support rail height chosen (350 - 600 mm) determines the vertical range available for experiments and microscopy components. The 7.74" throat depth, or distance from the optical path to the support rail, provides a large working space for experiments. Components attach to the body by way of either a linear dovetail on the support rail, or a circular dovetail on the epi-illumination arm (on certain models). Please see the Microscope Dovetails tab or here for further details.
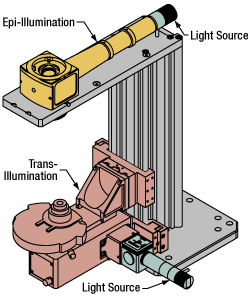 Click to Enlarge
Click to EnlargeIllumination with a Cerna microscope can come from above (yellow) or below (orange). Illumination sources (green) attach to either.
Illumination
Using the Cerna microscope body, a sample can be illuminated in two directions: from above (epi-illumination, see yellow components to the right) or from below (trans-illumination, see orange components to the right).
Epi-illumination illuminates on the same side of the sample as the viewing apparatus; therefore, the light from the illumination source (green) and the light from the sample plane share a portion of the optical path. It is used in fluorescence, confocal, and reflected light microscopy. Epi-illumination modules, which direct and condition light along the optical path, are attached to the epi-illumination arm of the microscope body via a circular D1N dovetail (see the Microscope Dovetails tab or here for details). Multiple epi-illumination modules are available, as well as breadboard tops, which have regularly spaced tapped holes for custom designs.
Trans-illumination illuminates from the opposite side of the sample as the viewing apparatus. Example imaging modalities include brightfield, differential interference contrast (DIC), Dodt gradient contrast, oblique, and darkfield microscopy. Trans-illumination modules, which condition light (on certain models) and direct it along the optical path, are attached to the support rail of the microscope body via a linear dovetail (see Microscope Dovetails tab or here). Please note that certain imaging modalities will require additional optics to alter the properties of the beam; these optics may be easily incorporated in the optical path via lens tubes and cage systems. In addition, Thorlabs offers condensers, which reshape input collimated light to help create optimal Köhler illumination. These attach to a mounting arm, which holds the condenser at the throat depth, or the distance from the optical path to the support rail. The arm attaches to a focusing module, used for aligning the condenser with respect to the sample and trans-illumination module.
 |
 |
 |
 |
 |
 |
 |
 |
| Epi-Illumination Modules | Breadboards & Body Attachments |
Brightfield | DIC | Dodt | Condensers | Condenser Mounting | Light Sources |
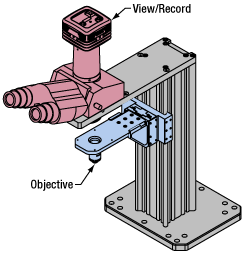 Click to Enlarge
Click to EnlargeLight from the sample plane is collected through an objective (blue) and viewed using trinocs or other optical ports (pink).
Sample Viewing/Recording
Once illuminated, examining a sample with a microscope requires both focusing on the sample plane (see blue components to the right) and visualizing the resulting image (see pink components).
A microscope objective collects and magnifies light from the sample plane for imaging. On the Cerna microscope, the objective is threaded onto a nosepiece, which holds the objective at the throat depth, or the distance from the optical path to the support rail of the microscope body. This nosepiece is secured to a motorized focusing module, used for focusing the objective as well as for moving it out of the way for sample handling. To ensure a light-tight path from the objective, the microscope body comes with a bellows (not pictured).
Various modules are available for sample viewing and data collection. Trinoculars have three points of vision to view the sample directly as well as with a camera. Double camera ports redirect or split the optical path among two viewing channels. Camera tubes increase or decrease the image magnification. For data collection, Thorlabs offers both cameras and photomultiplier tubes (PMTs), the latter being necessary to detect fluorescence signals for confocal microscopy. Breadboard tops provide functionality for custom-designed data collection setups. Modules are attached to the microscope body via a circular dovetail (see the Microscope Dovetails tab or here for details).
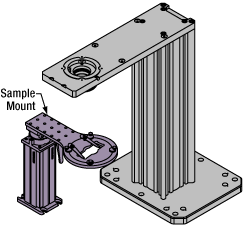 Click to Enlarge
Click to EnlargeThe rigid stand (purple) pictured is one of various sample mounting options available.
Sample/Experiment Mounting
Various sample and equipment mounting options are available to take advantage of the large working space of this microscope system. Large samples and ancillary equipment can be mounted via mounting platforms, which fit around the microscope body and utilize a breadboard design with regularly spaced tapped through holes. Small samples can be mounted on rigid stands (for example, see the purple component to the right), which have holders for different methods of sample preparation and data collection, such as slides, well plates, and petri dishes. For more traditional sample mounting, slides can also be mounted directly onto the microscope body via a manual XY stage. The rigid stands can translate by way of motorized stages (sold separately), while the mounting platforms contain built-in mechanics for motorized or manual translation. Rigid stands can also be mounted on top of the mounting platforms for independent and synchronized movement of multiple instruments, if you are interested in performing experiments simultaneously during microscopy.
 Products Home
Products Home




































 Trans-Illumination: Brightfield, Oblique, & Others
Trans-Illumination: Brightfield, Oblique, & Others