Investigating Biofilm Using OCT

Please Wait
Biofilm Structure at the Mesoscale Investigated Using Spectral Domain OCT
Featured Researchers:
M. Wagner, D. Taherzadeh, C. Haisch, and H. Horn
Biofilms, the dominant form of microbial life, are structured communities of microorganisms that adhere to surfaces in aquatic environments. Although there are various established methods for studying biofilm constituents and their distribution at the microscopic level [e.g., confocal laser scanning microscopy (CLSM), scanning transmission X-ray microscopy (STXM), and transmission electron microscopy (TEM) to name a few], these techniques are limited to providing localized information as opposed to unveiling global structural properties. To gain information on mesoscale biofilm structure, other techniques such as magnetic resonance microcopy (MRM) and magnetic resonance imaging (MRI) have been used. However, MRM and MRI systems are highly complex, have large footprints, and can be costly from an administrative and instrumental standpoint.
Figure 1. Top: 3D reconstruction of a 134-day-old heterotrophic biofilm (white) cultivated on a glass slide for three different flow conditions: laminar (re = 1000), transient (Re = 2500), and turbulent (Re = 4000). The red orthogonal slices were added to the 4 mm x 4 mm x 1.6 mm images to highlight the porosity of the biofilm structure. Bottom: Equidistant OCT A-scans (4 mm x 1.6 mm) showing cross-sectional views of the biofilm structure for the same three hydrodynamic flow conditions shown in the top frames. Recently, an alternative imaging modality has surfaced for studying biofilm structures in the millimeter range: optical coherence tomography (OCT). Wagner et al. have used a Thorlabs OCP930SR Spectral Domain Optical Coherence Tomography system to study the mesoscopic structure of cultivated heterotrophic biofilms in situ. The compact, mobile, easy-to-use Thorlabs OCT system is capable of providing nondestructive, high resolution (<20 μm) images of the biofilm structure at mesoscale. Three-dimensional OCT images measuring 4 mm x 4 mm x 3 mm were obtained in less than 2 minutes at a resolution of 8 μm x 16 μm x 4 μm (xyz, 250 A-scans). The biofilms were cultivated on glass slides in a custom-made funnel system. Flow rates were adjusted to achieve laminar (Re = 1000), transient (Re = 2500), and turbulent (Re = 4000) flow environments. The isosurface projections shown in the first row of Fig. 1 show the mesoscopic biofilm structure for each flow regime. Biomass and particulate materials within the biofilm matrix are visible. Red slices are inserted orthogonal to the biofilm support to highlight the porous structure. From the figure, it is evident that biofilms cultivated in laminar and transient flow environments are more porous than those cultivated in a turbulent environment. Furthermore, volumetric porosity calculations indicate that the unoccupied volume in these non-chaotic-flow environments is nearly double that of the turbulent environment, thereby indicating the presence of different structural properties in the latter. The bottom 5 x 3 frames of Fig. 1 show a series of equidistant, 4 mm x 1.6 mm A-scans. The structure of the biofilm cultivated at Re = 1000 and Re = 2500 consists of aggregates of varying sizes and levels of connectivity separated by voids ranging in size from microns to millimeters. Furthermore, for the laminar and transient flow, the OCT images reveal a layered structure on top of which an open structured biofilm developed. In contrast, for the biofilm cultivated in turbulent flow, the mesoscopic structure is dense, appearing visually homogeneous and lacking significant porosity. There is, however, variation in surface structure. By quantifying the differences in porosity among biofilms cultivated in varying hydrodynamic conditions, researchers can improve their understanding of mass transfer effects inside biomass. Based on the data presented here, Wagner et al. believe that the mesoscopic biofilm structure enables advective transport of nutrients through the pores and along the open-structured biofilm-bulk fluid interface. The results shown here indicate that OCT is a promising imaging modality for bridging the gap between micro and macroscale biofilm studies. |
References:
1) M.Wagner, D. Taherzadeh, C. Haisch, and H. Horn, Biotechnol and Bioeng. DOI: 10.1002/bit.22864
| Posted Comments: | |
| No Comments Posted |
 Products Home
Products Home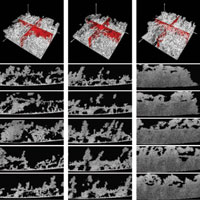
 Biofilm Structure Investigated using OCT
Biofilm Structure Investigated using OCT